UO biologists, with the help of zebrafish, now have loose ties to Star Trek, Heroes and Spider-Man.
Those popular science fiction productions illustrate a cultural fascination with super-human organ regeneration, inspired by the reality that many backboned animal cousins of humans can naturally restore lost organs, including bone.
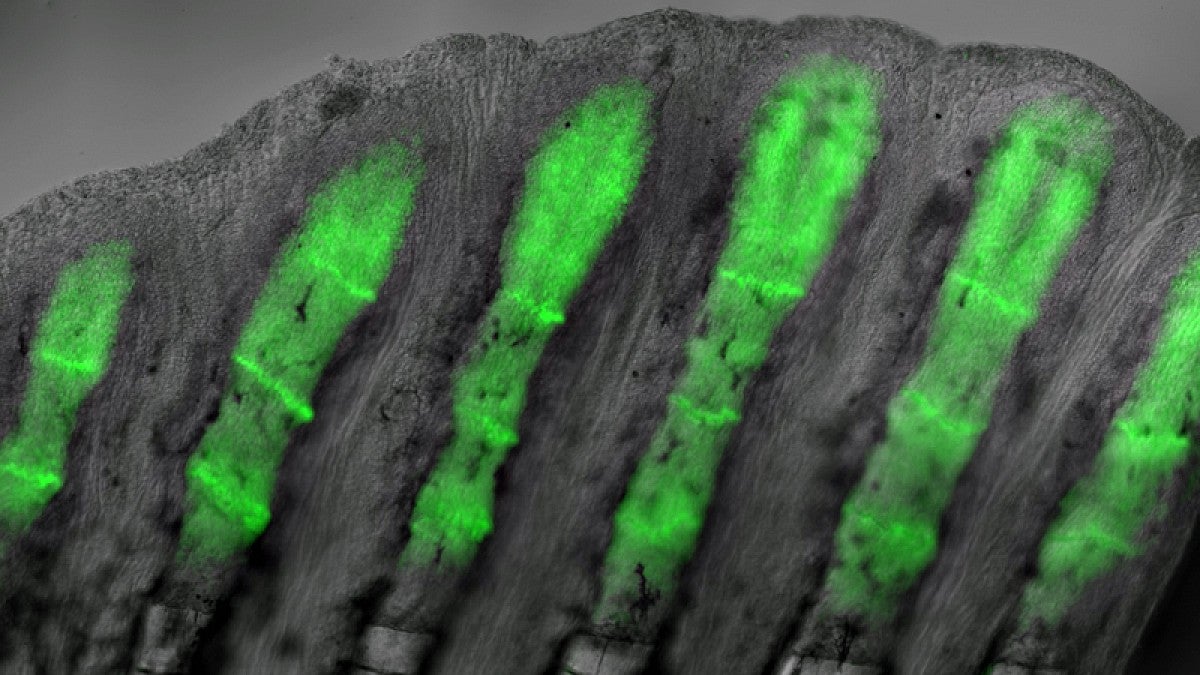
In the UO lab of Kryn Stankunas, researchers have obtained one of the clearest pictures yet on how adult zebrafish master the feat. In a paper this week in the journal Development they report how the small fish are able to regrow amputated tail fins with a precisely organized skeleton of bony rays within two weeks.
“The orderly reconstruction of zebrafish fins is amazing to see,” said Stankunas, a professor in the Department of Biology and member of the Institute of Molecular Biology. “Zebrafish fins, which are akin to our limbs, regenerate perfectly. The zebrafish bony rays rebranch just like the original structure. This would be like losing your arm and watching it progressively regenerate complete with a hand and fingers — all the bones restored in their original configuration.”
The findings will not lead to humans regrowing lost limbs, Stankunas said, but understanding the processes of regeneration in related vertebrate organisms may eventually lead to new targeted therapeutic strategies to improve the repair of broken bones.
“The mechanism — how the skin and bone cells dynamically move and interact using cell signaling — is elegant and unexpected, broadening the project’s impact on regenerative medicine,” Stankunas said.
The research team used genetically modified zebrafish that produce a fluorescent protein, which helped them identify skin and bone cells that respond to a specific type of cell-to-cell communication called hedgehog signaling.
Clusters of specialized skin cells moved over reforming bones, escorting the cells into positions to form individual bones of a branched skeleton. These cells produce a protein called sonic hedgehog, which interacts with bone-building cells to promote bone patterning.
The researchers documented the regenerative process using advanced microscopy techniques.
“We could see that the bone cells responding to the skin-produced sonic hedgehog become physically attached to the migrating skin cells,” said Scott Stewart, a research professor at the Institute of Molecular Biology who co-led the project. “The pathway is quickly turned off but the now-split groups of bone cells will then form two separated mature bony rays connected at a branch point.”
To define the functions of the hedgehog signaling pathway, the researchers used a chemical inhibitor to turn off hedgehog signaling in their experimental fish. With the signaling blocked, the skin and bone cells failed to interact, and the fin regenerated with stick-like rays rather than forming a branched skeleton.
“Hedgehog signaling is absolutely required for branching and not essential for any other aspect of regeneration,” Stankunas said.
Benjamin E. Armstrong, who earned a doctorate in biochemistry in 2016, did most of the experiments in the research, which was supported by the National Institutes of Health.
—By Jim Barlow, University Communications