The sun, a class G2V yellow dwarf star to the more scientific among us, rises in the small town of Creswell, south and just east of Eugene, about thirty seconds earlier than it does on the University of Oregon campus. The thin line of morning moves across Oregon at some 500 miles an hour, but the light from our local sphere of unimaginably hot plasma has taken just eight minutes and nineteen seconds to travel nearly 93 million miles to the Willamette Valley. The sun is Earth’s furnace, light bulb, power source—the sun is quite literally Earth’s everything, as it happens, because when the nuclear fusion process that is now converting some 680 million tons of hydrogen into helium each second runs out of fuel, it’s lights out for us. We have about five billion years to prepare.
Most of the energy that reaches Earth is reflected back into space. Much of what’s left is consumed in the process of evaporating water from the oceans. Only a small fraction of the energy remains: When the sun has risen to high noon and it is shining directly on, say, one of the lawns in the Lokey Science Complex, about 1,000 watts are being deposited on each square meter of surface. The grass is using that energy to convert carbon dioxide and water into organic compounds, mainly sugars, through photosynthesis. It is calculated that the rate of energy captured by photosynthesis on Earth is 100 terawatts at any given moment, which is about six times more than the current rate of power consumption of all human civilization.
Photosynthesis and the resulting creation of nutrients that feed the plants and eventually feed virtually all other life forms, either directly or indirectly, is the way nature captures and stores solar energy. A certain group of those life forms, human beings, have discovered effective inorganic ways to capture solar power, but have only the most inefficient ways to store it once captured. This makes the use of currently arriving solar power problematic in an age when said human beings are rapidly using up previously banked solar power—which is the true definition of fossil fuels: solar power captured long ago by photosynthesis and locked away in the spinning vault of our planet.
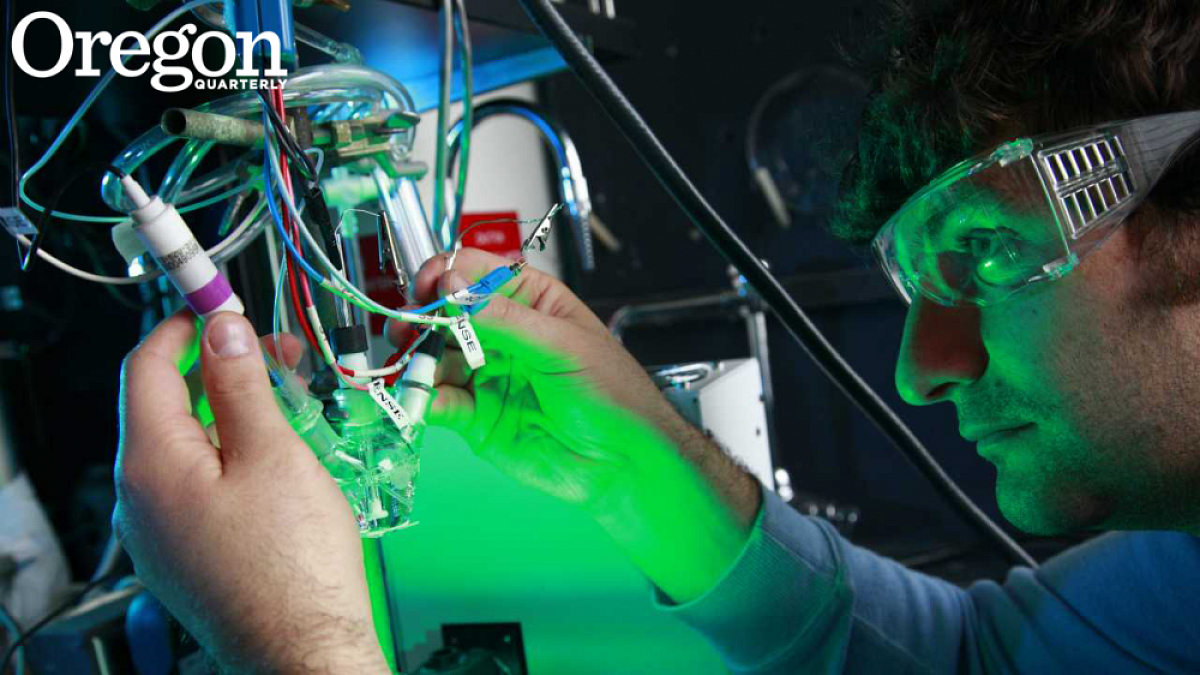
Thirty-year-old Shannon Boettcher ’03 (pronounced Bet-cher), a UO chemist and expert in materials science, believes we can—and, more importantly, will—find a solution to efficient, scalable, and affordable capture and storage of solar energy. And it will happen, he believes, in his lifetime—“unless we fail completely, or we develop something better.” The former, as even those of us with the sunniest dispositions can agree, would be bad.
* * *
Boettcher was born in Santa Cruz, California, and moved at age eleven with his family to Creswell when his mother, biochemist Rebekka Wachter, PhD ’96, decided to get her doctorate at the UO. His father, Jim, ran a computer repair business and harbored dreams of being a gentleman farmer, so the Boettchers moved onto fifteen acres in the country, with a corresponding complement of animals and tractors and chores. His father was a major DIY guy, and Boettcher grew up with a fascination for how things work. Even in the thick of graduate school, his mother was always home for dinner and to do “mom stuff” with Boettcher and his younger sister, Sara—a level of having one’s act together that Boettcher only began to appreciate as he was working long hours toward his own doctorate.

“Going to a small country school,” he says, “you don’t get caught up in all the noise about choosing the ‘right’ college. I didn’t have any of that. I just thought, ‘The UO is ten miles away, it’s in-state tuition, and they’ll give me a scholarship—why wouldn’t I do that?’ I couldn’t be happier about the way it turned out.”
Boettcher chose chemistry as his major and soon was involved in undergraduate research in materials science, particularly with electronic materials. He studied with Professor Mark Lonergan ’90, who would eventually become his colleague in the UO Department of Chemistry, and won the top award given by the Robert D. Clark Honors College for his undergraduate thesis. After he graduated in 2003, he went on to earn his PhD at the University of California at Santa Barbara (UCSB), home to Alan J. Heeger, a Nobel Prize–winner in the science of energy-conducting polymers. In 2008, Boettcher moved on to postdoctoral training at the legendary California Institute of Technology. Along the way, the honors and awards kept coming, from Goldwater Scholar at the UO to National Science Foundation Graduate Research Fellow at UCSB and Kavli Nanoscience Institute Prize Postdoctoral Fellow at Cal Tech.
But the Willamette Valley was calling, and in 2010 Boettcher returned to join the UO faculty and helped form a group of chemists, physicists, and engineers “committed to addressing the fundamental and significant materials challenges associated with the capture and storage of solar energy.” In other words, to change everything.
* * *
Storage has long been the problem with solar and wind power. Even after more than two centuries of development, the best modern batteries are relatively inefficient, expensive, and usually filled with hazardous materials. Your shiny new laptop is a perfect example: On day one, the battery charge lasts for six or eight or even ten hours—but a couple years down the road the charge is gone in two or three hours, and, should you decide to replace the battery, you discover that it costs $200 or more. And that’s to power a device that uses less energy than a sixty-watt light bulb.
“This is a very compelling problem to work on,” says Boettcher. “Conventional solar panels use slices of silicon crystals, similar to those in your computer, which absorb sunlight and generate excited electrons. These panels very efficiently siphon these electrons into a wire, generating current and voltage to run a light or charge a battery or power a motor. We have reached the point where these panels are remarkably efficient, and we are approaching the fundamental limits of that efficiency. That’s a good thing in one sense, but the challenge is that if you can’tstore that electricity for when the sun isn’t shining, it becomes very difficult to power a plane or a factory or a city—anything that requires power twenty-four hours a day.”
He recalculates. “Did I say ‘challenge?’ I should say huge challenge. Batteries to store these excited electrons are expensive to put together, and it’s not clear, although progress is being made, that there will ever be a scalable, practical pathway to using batteries to store the density and amount of energy required for large applications.”

“If you look at today’s options,” concludes Boettcher, “they just don’t seem to be workable for even modest storage. And the needs will be enormous as this industry ramps up. Today, solar provides one-tenth of 1 percent of our electricity. It’s minuscule at this point. And electricity provides just one-fifth of our total energy use. So simply building all the solar panels we would need will take a huge capital investment, and quickly you’ll hit the storage limit and any extra energy you generate won’t do you any good. The same is true of wind power. But to curb the negative effects of our CO2 generation we must find a way to get all or most of our energy from renewable sources. We have to solve this problem.”
Did he say “huge challenge?” He should say gigantic challenge. But scientific research would be a pretty depressing gig if you weren’t an optimist, and Boettcher qualifies.
His research centers on finding a way to use the electrons that are generated in a solar-reactive material and, rather than funneling them into a wire to charge a battery or run a motor, to instead drive an electrochemical reaction that directly creates a stable fuel that can be stored and used at any time. To be, in effect, as smart as your basic dandelion. Indeed, “artificial photosynthesis” is the catch phrase for this branch of solar materials science.
“The easiest way to do this,” according to Boettcher, “is to take a water molecule and split it into hydrogen and oxygen. The hydrogen becomes a compressible, liquefiable, burnable fuel, which can be put into fuel cells to make electricity, or used to make liquid biofuels, or to make liquid methanol as a gasoline substitute. There are lots of things that can be done with hydrogen, but always the key is that they must be done at a really competitive cost. How do we do this without complex, expensive engineering?
“We can do just about anything if we spend enough money, but with energy you can’t charge much more, if any more, than competitive fuels. It’s very clear in this political climate that that’s an impossibility. So, can we design a system that’s cheap enough? If we don’t do this in my lifetime, even in the next twenty years, it will mean one of just two things: we failed completely or we developed something even better. I’m an optimist, and I believe we will figure out a way to solve the energy problem.”
One cause for Boettcher’s optimism is his sense that the major energy corporations and science-based multinationals, although still deeply dug into their defensive trenches, are not now active enemies of so-called “alternative” energy. In fact, one of those companies, DuPont, recently named Boettcher one of eighteen recipients worldwide of the company’s Young Professors Grants, which honor promising researchers at the outset of their careers.
“The feeling I get from industry folks,” Boettcher says, “is that they realize this is a big problem and answers are critical. They understand that they will have to change eventually, but they also realize that they have hundreds of billions of dollars already invested in capital infrastructure for finding, processing, and selling fossil fuels. They tend to be very conservative and they are hugely profitable at what they do now, so they are not going to change overnight. That leads to an investment in renewable energy that is a little disappointing. It’s basically a drop in the bucket.
“Look at a company like BP: they’ve made some very good investments in renewables, but if you consider a $500 million investment over five or ten years in the context of their annual profits, that’s not very much by their standards. It seems to me that it would be in the energy companies’ strategic interests to invest much more than they are in renewable energy, but so far they don’t see it that way. It’s their future, too!”
Boettcher hopes that we don’t repeat what he sees as the real tragedy: the 1980s.
“We had a big push in the late ’70s for renewable energy,” he says, “but that just died in the ’80s. There was very little basic research, very little investment. We lost twenty years.”

“There is one camp that always claims, ‘Americans innovate better than anyone else in the world,’” Boettcher says. “I don’t believe that. I think there are smart people everywhere, which is a great thing. I’m excited that the Chinese are serious about making solar energy cheaper. I’m far less excited about the reasons why their panels are cheaper: they pay their workers much less, they undervalue their currency, and they burn coal for their factories without any environmental restrictions. They aren’t winning because they are innovating better; they are winning because they have unequal costs. That worries me, because if our companies can’t compete, we won’t have a solar industry here.”
Boettcher and his fellow researchers around the country face a different sort of energy shortage that poses nearly as big a challenge as the questions they are working to answer: the precipitous decline in federal funding for research.
First, a basic primer on the career of a research scientist: 1) Young woman or man discovers a love for science and research. 2) Studies for a decade or more to learn how to be a good researcher. 3) Lands a job in a research lab to do what he or she loves. 4) Rises to a position where she or he can start his or her own lab. 5) Immediately stops doing what she or he loves to begin an endless chase for money. In scientific research, reaching the peak of your profession means you essentially stop doing your profession.
Oversimplified, yes, but not by much.
“Funding rates for research grant applications, which take months of work to put together,” Boettcher explains, “are now around one in ten. Months of preparation and sixty- to eighty-hour work weeks, with a 10 percent chance of success. Which makes it very difficult to innovate, because any idea that is ‘too new’ will be considered risky and won’t get funded, so no one will try. Most people don’t realize the scale of this problem—we as a country will have a hard time maintaining a competitive edge without research and discovery. Basic science is vital, even when the impact isn’t yet clear.”
It is clear that the difference a small shift in priorities could make would be night and day—to say nothing of solar and no solar. For 2012, the National Science Foundation has requested a budget of $7.7 billion, which is 61 percent of the total federal spending for nonmedical scientific research. In comparison, Americans spend more than $38 billion per month on gasoline. One month of the war effort in Afghanistan cost the federal government $6.7 billion in 2010.
So what is a young research leader to do when he just wants to help reshape the world’s energy future? Go back to lessons learned on fifteen acres in Creswell:
“I just stay optimistic,” Boettcher says, “just get to work and just do my best. If we can develop these new materials for affordably capturing and storing solar power, everything is going to change in a big way.”
One thing is certain amid all the challenges: the G2V yellow dwarf star will come up in the morning.
—By Todd Schwartz
Todd Schwartz ’75 is a Portland-based writer who, as a nine-year-old, was promised at the 1962 Seattle World’s Fair that by now we would already have had sun-powered flying cars—and is wondering what the hell happened.