Just as Marian Hettiaratchi begins her new job this month as a bioengineer at the Phil and Penny Knight Campus for Accelerating Scientific Impact, a research project that lays the groundwork for her UO lab has landed in a major journal.
Her paper in the Jan. 3 issue of Science Advances takes a big step toward improving the effectiveness of spinal fusion procedures and repairing broken or defective bones.
In the preclinical study, done during her graduate work Georgia Tech, her team drastically reduced undesired bone growth outside of a targeted repair area by using a mixture of bone morphogenetic proteins, which promote tissue and bone development, and microparticles made of heparin, a drug widely used as a blood thinner.
The accomplishment is a proof of concept that shows the natural bone protein, known as BMP, can be merged into a heparin-like biomaterial for safer delivery. Given alone in high doses, as has been the practice in human treatments, BMP leakage has led to soft tissue inflammation and abnormal bone growth.
At her new UO lab, Hettiaratchi will try to synthesize a heparin-like substance that can deliver BMP while avoiding the potential side effects of heparin, none of which have been seen in the work with rats. Her approach also uses a nanofiber mesh tube unveiled in 2011 by Robert Guldberg, who left Georgia Tech in 2018 to become executive director of the Knight Campus. He is a co-author on the new paper.
“I am planning on applying this approach more broadly to healing other injuries and diseases since proteins are important in every system in the body,” Hettiaratchi said. “My lab's objective is to create biomaterials that can locally deliver proteins to sites of injuries with high precision to accelerate tissue repair.”
She began exploring the use of heparin microparticles to deliver BMP while a doctoral student at Georgia Tech under the mentorship of Guldberg and co-author Todd McDevitt.
For the new study, Hettiaratchi and colleagues fed their earlier results from experiments done in rats and test tubes into computer simulations to guide how they could adjust their heparin-based approach in animal testing with levels of BMP comparable to dosages required in human bone-repair procedures.
“We focused on using doses that were more clinically relevant,” Hettiaratchi said. “In humans, the typical treatment uses 0.1 to 0.2 milligrams of BMP per kilogram of body weight, so we used the same amount in the rats. Most research done in rats uses 10 times less BMP to repair bone, which isn’t comparable to what’s done in humans and doesn’t exhibit the side effects of a clinical BMP dose.”
Two different strengths of the combination used in the study reduced unwanted bone growth by 40 to 50 percent.
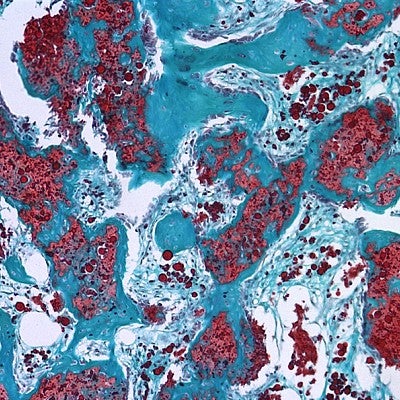
That mixture was injected into the nanofiber mesh tube, which had already been inserted into leg bone defects in the rats. The approach was designed to replace a collagen sponge, which allowed BMP in human bone repairs to leak outside of targeted areas.
“The problem with healing large bone defects clinically is that the BMP delivered using collagen sponges results in abnormal bone formation because the drug doesn’t stay on the material,” Hettiaratchi said. “Our new material retains much more of the BMP, keeping it localized. You don’t get bone formation outside the targeted area.”
Hettiaratchi joined the UO after completing a postdoctoral fellowship at the University of Toronto, where she began her quest to create a synthetic biomaterial that will remain in a targeted area and bind only to BMP, allowing it to be released into the tissue at a desired rate.
Co-authors with Hettiaratchi, Guldberg and McDevitt, who is now in San Francisco at the Gladstone Institute of Cardiovascular Disease and the University of California, were Laxminarayanan Krishnan, Tel Rouse and Catherine Chou, all of Georgia Tech’s Parker H. Petit Institute for Bioengineering and Bioscience.
The National Institutes of Health and Armed Forces Institute of Regenerative Medicine supported the project.
—By Jim Barlow, University Communications


